On October 18, 2024, LUCI, China’s first adjustable intracranial stent retriever developed by Grand Pharma, received its medical device registration certificate from the China National Medical Products Administration (NMPA), marking a significant milestone for the Group in advancing neurointerventional technology within precision interventional diagnosis and treatment for cerebro-cardiovascular conditions.
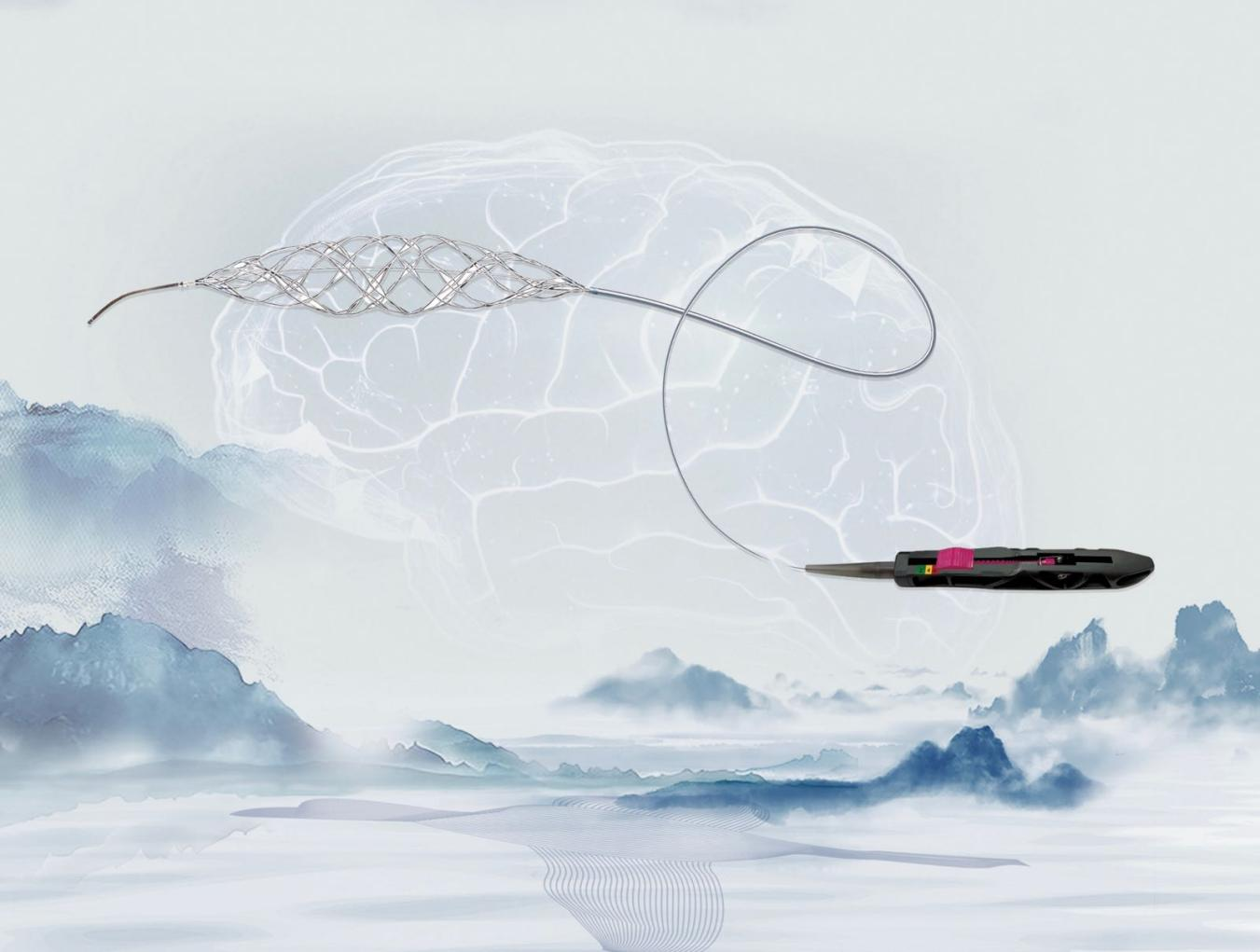
LUCI, China’s first adjustable intracranial stent retriever, adapts flexibly to varying blood vessel diameters by adjusting the stent’s diameter during thrombectomy. This feature enhances the stent’s ability to embed within the thrombus, thereby improving procedural effectiveness, minimizing vascular damage, and enhancing overall safety. Clinical data indicate that LUCI demonstrates clinical efficacy and safety comparable to the control group in treating acute ischemic stroke, with a successful recanalization rate of up to 98.3% and a single-pass recanalization rate of up to 68.9%.
With an aging population and an increasing incidence of stroke among younger people, China now faces one of the world’s highest lifetime stroke risks and the greatest stroke-related disease burdens. After two decades of progress in neurointerventional therapies and continuous product innovation and iteration, a growing number of Chinese patients are expected to benefit from safer and more effective neurointerventional treatments.
Grand Pharma has long been dedicated to advancing cerebro-cardiovascular precision interventional diagnosis and treatment. Guided by the principle of “non-invasive intervention,” the Group has developed a comprehensive portfolio of high-end medical devices, focused on three key areas: vascular path management, structural heart disease, and electrophysiology and heart failure. Currently, the Group’s cerebro-cardiovascular precision interventional diagnosis and treatment segment includes 14 products, with 9 approved and available in China. Grand Pharma has also built “passive + active” innovative device platforms, forming an R&D and production framework with two centers in China and multiple facilities abroad, reinforcing its goal of creating a leading platform for cerebro-cardiovascular precision interventional diagnosis and treatment in both China and the global market.
###
LATEST NEWS
Lei Yunshang Herbal Paste Festival: A Perfect Fusion of Tradition and Innovation, Ushering a New Trend in TCM Wellness
2024-11-12
Grand Shopping Center’s Platinum VIP Event Achieves Over RMB 135 Million in Single-Day Sales Across 100 Brands
2024-11-12
Sinclair Sets Sail at WEM 2024 Global Medical Aesthetics Summit Leading the Future of Global Anti-Aging
2024-11-12
Grand Pharma’s LUCI: China’s First Adjustable Intracranial Stent Retriever Receives Medical Device Registration Certificate
2024-11-12
Second Generation of ELLANSÉ® Makes Stunning Debut at Sinclair’s New Product Launch
2024-07-10
Grand Pharma brings heavyweight products to the World Health Expo
2023-04-12