Editor’s note: Recently, Huadong Medicine Co., Ltd. (“Huadong Medicine”), a member of China Grand Enterprises, completed the acquisition of all shares of Sinclair, a UK-listed aesthetic medicine company, which became a wholly-owned subsidiary of Huadong Medicine Aesthetic Medicine Investment (Hong Kong) Co., Ltd. During a period of about 5 months, the company completed work from preliminary contact, negotiation on intentions, design of deal structure, signing of an agreement of intent and agreement on the arrangement of the project, as well as relevant filings with government bodies, fund arrangements and other necessary arrangements. The successful completion of the entire transaction in such a short time highlights the efforts and determination of Huadong Medicine’s international development.
Below is an extract of the relevant information released on the “Official Account of Huadong Medicine Investor Relations Management”:
On the evening of November 8, 2018, Huadong Medicine issued the Announcement on Completing the Acquisition of the Entirety of British Sinclair Pharma plc Shares with a Cash Offer. Sinclair has now become a wholly-owned subsidiary of the company’s wholly-owned sub-subsidiary Huadong Medicine Aesthetic Medicine Investment (Hong Kong) Co., Ltd., and will be included in the consolidated statements of the company from November 2018.
Acquisition process
During the entire agreement arrangement, the company has actively promoted transactions in accordance with established plans and made timely disclosures of information pursuant to the requirements of the relevant laws and regulations of China and the United Kingdom, as well as the relevant M&A regulations. Across a time period of about 5 months from July 5, 2018, when the company announced the preparatory acquisition of all shares of Sinclair, until the present, the company completed work ranging from preliminary contact, negotiation on intentions, design of transaction structure, signing of the agreement of intent and finalizing project agreement arrangements, as well as relevant filings with government bodies, fund arrangements and other preparations. The successful completion of the entire transaction in a short period of time highlights the efforts and determination of Huadong Medicine’s international development.
Being optimistic about the market potential and growth prospects of the aesthetic medicine industry in the context of consumption upgrading, the company began work on this arrangement many years ago. The holding subsidiary Huadong Ningbo Medicine Co., Ltd. is a leading company in aesthetic medicine products sales in China, with outstanding marketing capabilities and a leading industry status. It has over 6 years’ experience in market operation and promotion experience in the field of aesthetic medicine. As an aesthetic medicine company that operates globally, Sinclair covers the entire industry chain from R&D to production and sales, and employs more than 200 people. Its technical advantages in products, brand influence, market position and growth potential, in addition to an excellent management team and synergy with the company’s existing aesthetic medicine business, are the core factors that made this acquisition possible for the company.
Future plans and prospects for Sinclair
Following the completion of the tender offer for the British Sinclair’s shares, the company will directly obtain the internationally leading aesthetic medicine product lines, international sales network and all intellectual property rights. The company will provide financial, technical and product support in addition to support for other other aspects of the platform. Once it enters the Chinese market in the future, Sinclair’s products will be in cooperation with this company’s existing YVOIRE products to realize Huadong Medicine’s new product and technology distribution, cooperation and technology promotion in the field of aesthetic medicine. At the same time, in the process of international development, the company will continue to accumulate development experience, cultivate a talent team with an international perspective, realize the mutual exchange of domestic and foreign related products, technologies and talents, optimize the company’s existing operation management system, enhance the company’s international capital operation capabilities and comprehensive modern management system, and finally realize the international innovative development of Huadong Medicine.
About British Sinclair Pharma
Headquartered in London, UK, Sinclair is a professional aesthetic medicine company with world-leading aesthetic medicine technology and global operations. Its business covers the entire industry chain from research and development to production and sales. It enjoys technical advantages of products, brand influence, market position and future growth potential, as well as an international management and operations team of more than 200 people.
1. Sinclair’s main products
Sinclair’s core products — Silhouette Soft and Ellansé Injectable Long-Acting Microspheres — feature industry-leading technologies. The products also include PerfectHA hyaluronic acid and Sculptra microspheres for agent sale in the European market. By building a diverse product portfolio, the company provides a richer, high-quality aesthetic medicine product experience and solutions for beauty and fashion-conscious people worldwide.
Silhouette Soft® and InstaLift™ suture
Ellansé® Biodegradable Microsphere Injection
Perfectha® Hyaluronic Acid Series

2. Registration and coverage of Sinclair products in major markets worldwide
Sinclair products have been registered and launched in more than 50 countries and regions around the world. Most of the products are registered in major aesthetic medicine markets including Europe, the United States, Brazil and South Korea. In the Chinese market, registration of Ellansé products has been carried out, and additional products are expected to be registered and launched to the market in 2019 and within 2-3 years thereafter.
Sinclair adopts the “direct sale + distributor” sales model and sells its products in 55 countries around the world. A self-operation model has been adopted in countries such as the United States, Brazil, South Korea, the United Kingdom, France, Spain, Mexico and Germany, and products are sold in the Middle East, Eastern Europe, Africa, Asia Pacific, Russia and Latin America (apart from Brazil) through 38 distributors.
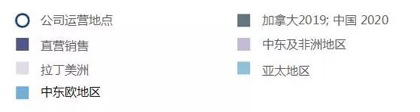
Sinclair’s European Business Layout
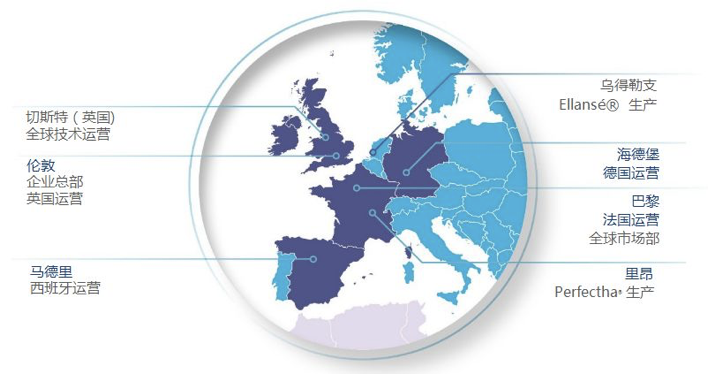
Sinclair’s Global Business Layout
LATEST NEWS
Lei Yunshang Herbal Paste Festival: A Perfect Fusion of Tradition and Innovation, Ushering a New Trend in TCM Wellness
2024-11-12
Grand Shopping Center’s Platinum VIP Event Achieves Over RMB 135 Million in Single-Day Sales Across 100 Brands
2024-11-12
Sinclair Sets Sail at WEM 2024 Global Medical Aesthetics Summit Leading the Future of Global Anti-Aging
2024-11-12
Grand Pharma’s LUCI: China’s First Adjustable Intracranial Stent Retriever Receives Medical Device Registration Certificate
2024-11-12
Second Generation of ELLANSÉ® Makes Stunning Debut at Sinclair’s New Product Launch
2024-07-10
Grand Pharma brings heavyweight products to the World Health Expo
2023-04-12